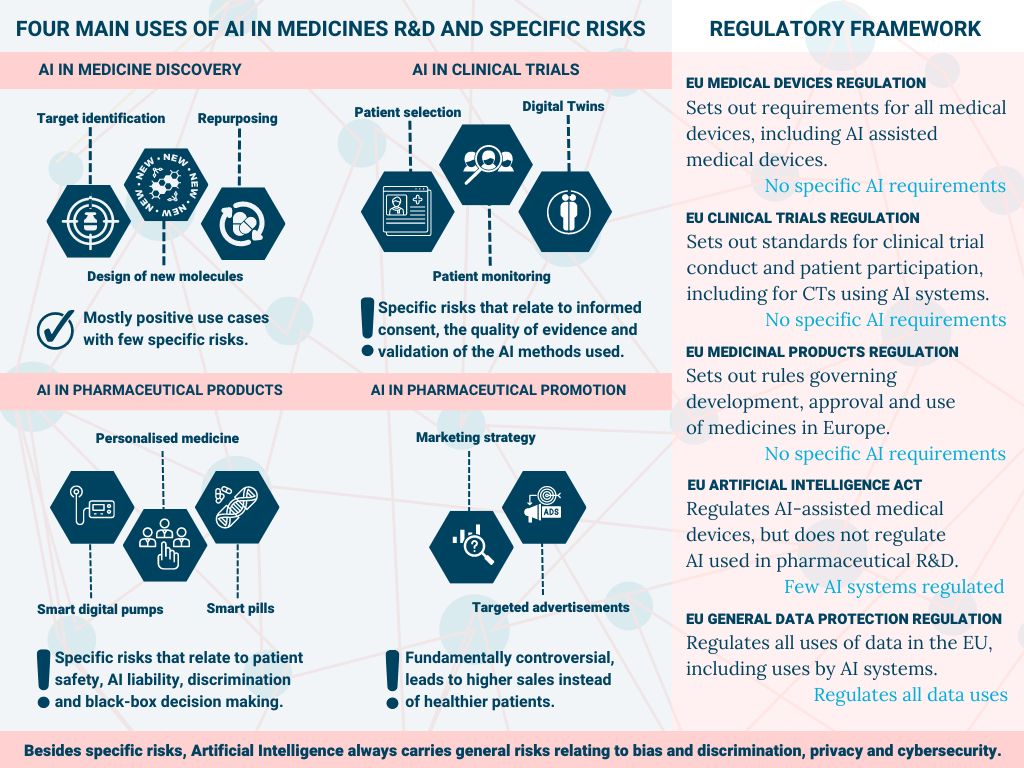
Artificial Intelligence in medicines R&D
This infographic illustrates the diverse range of potential uses of AI in medicines R&D and risks involved. Some of the uses, such as ‘digital twins’ (using digital copies of biological entities to predict clinical outcome in different treatment scenarios) are still speculative and it remains unclear what their contribution to reliable evidence generation can be. Other uses, such as identifying opportunities for the repurposing of medicines, are more established and likely to have a positive impact on the discovery of new treatment opportunities. On the other end of the spectrum, AI for the optimisation of sales and marketing, albeit effective, is highly controversial. As pharmaceutical promotion does not improve prescribing practices, but may worsen them (read our report Fact or Fiction), use of advanced analytics and AI to optimise pharmaceutical promotion will not lead to public health gains. The wide array of uses should be closely monitored and explored, and its risks systematically identified and addressed through appropriate regulatory and policy frameworks.
The various elements that comprise medicine R&D, including clinical trials, medicinal products, medical research, and AI and data, are regulated through different EU legal frameworks with specific national ‘member state competencies’. The fragmented and sometimes outdated legal landscape currently falls short; none of the EU medical regulations (i.e., on clinical trials, medicinal products and medical devices) contain any specific regulatory requirements for AI-assisted-clinical trials, -medicinal products, and -medical devices, respectively. On the other hand, the new EU AI regulation, which is still under negotiation, mostly limits regulation to systems that are classified as a medical device. These gaps must be addressed, as some uses of AI in medicine R&D carry risks to standards of evidence, and safety and fundamental rights of people. Moreover, the European Medicines Agency and National Competent Authorities should take a leading role in developing comprehensive standards and guidance which steer the use of AI in this sector in the right direction. These two essential avenues of action must be taken to realise trustworthy AI that advances the discovery of effective new medicines, increases the generation of high-quality evidence, and makes a real contribution to the health of patients.
Download the infographic here.